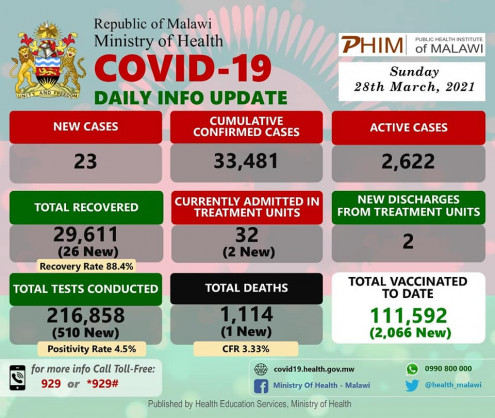
Malawi has registered 23 new COVID-19 cases, 26 new recoveries and one new death. Of the new cases, 21 are locally transmitted: 11 from Blantyre, nine from Lilongwe, and one from Kasungu District while two of the new cases are imported and were identified at Mwanza border during routine screening: one each from Blantyre and Zomba Districts. One new death was registered from Kasungu District. To the families that have lost their loved during this pandemic, may you find peace, hope and love during this difficult time. May the souls of the departed rest in peace.
Cumulatively, Malawi has recorded 33,481 cases including 1,114 deaths (Case Fatality Rate is at 3.33%). Of these cases, 2,114 are imported infections and 31,367 are locally transmitted. Cumulatively, 29,611 cases have now recovered (recovery rate of 88.4%) and 134 were lost to follow-up. This brings the total number of active cases to 2,622. Two cases were hospitalised while two were discharged. A total of 32 active cases are hospitalised: 10 in Blantyre, seven in Lilongwe, three each in Mchinji and Salima, two each in Thyolo and Zomba, and one each in Mzimba North, Mangochi, Dedza, Rumphi, and Ntchisi Districts. On testing, 510 COVID-19 tests were conducted. Of these, 275 tests were through SARS-CoV-2 Antigen Rapid Diagnostic test while the rest were through RT-PCR. The positive cases out of the total, translates to a positivity rate of 4.5%.
Cumulatively, 216,858 tests have been conducted in the country so far. On COVID-19 vaccination, cumulatively 111,592 people have been vaccinated in the country with 2,066 being vaccinated.
As we are still experiencing community transmission of COVID-19, I would like remind the public on the COVID-19 symptoms to facilitate the early care seeking when we experience the symptoms. It has to be noted that COVID-19 affects people in different ways. Most infected people will develop mild to moderate illness and recover without hospitalization. The most common symptoms include fever, dry cough, and tiredness, while in some patients it presents with aches and pains, sore throat, diarrhoea, conjunctivitis, headache, loss of taste or smell, a rash on skin, or discolouration of fingers or toes. When one has these symptoms, there is need to have a COVID-19 test. If one experiences the following serious symptoms ofdifficulty breathing or shortness of breath, chest pain or pressure, loss of speech or movement, immediate medical attention is needed. It is important to note that early care seeking lead to positive treatment outcomes.
Lastly, on COVID-19 Vaccination, let me emphasize that the AstraZeneca vaccine which we are using is safe as it has less or fewer side effects. Most of them are minor and self-limiting and these include pain or soreness on the injection site, fever, headache, muscle or joint pain, fatigue or nausea. These will usually go away without any need of hospital treatment.People receiving the vaccine are informed on the possible side effects of the vaccine including those that may require to be immediately reported to the health authorities or facilities. Those that have or will be receiving the vaccine are encouraged to report to nearest health facility any adverse effects following immunization or call toll free number 929. It is important to note that reporting adverse reactions of COVID-19 vaccine supports continuous monitoring of the safe and effective use of the vaccine in our country.
No one is safe until everyone else is safe. Protect yourself. Protect your loved ones. Protect everyone. Call toll free 929.
Hon. Khumbize Kandodo Chiponda, MP
MINISTER OF HEALTH
CO-CHAIRPERSON – PRESIDENTIAL TASKFORCE
Distributed by APO Group on behalf of Ministry of Health and Population, Republic of Malawi.
Source: Apo-Opa
Did you find this information helpful? If you did, consider donating.
